Four weeks have passed since I wrote my last blog about gold’s evolving role in diagnosing COVID-19, the pandemic that is affecting us all currently. In that time much has changed, and I wanted to update you all on our thoughts of where things stand in mid-April.
The UK is home for me, and the subject of COVID-19 diagnostics has never been far away from the front pages. The UK government has repeatedly championed the important role antibody testing will play in getting our lives back to normal, as this offers a route to tell whether someone has been infected by COVID-19 and can, therefore, return to work after an appropriate recovery period. As I described previously, these tests can be performed in a centralised laboratory, but this can be a slow and costly process. Having access to a portable, rapid test for point of care settings such as a pharmacy or doctor’s surgery (or potentially even home use) would represent a huge step forward in the fight against COVID-19.
However, achieving this has turned out to be far from straight forward. The UK government has evaluated a number of commercially available Lateral Flow Assays and have yet to deem any suitable for widespread use.1 No details of these studies are yet available, but their conclusion isn’t perhaps a huge surprise. COVID-19 has only existed for a few months, meaning scientists have not yet had time to study the disease and develop all of the required diagnostic tools at a sufficient level of accuracy. The much quoted phrase ‘a bad test is worse than no test at all’ is an absolute truth; falsely determining someone has (or has not) contracted COVID-19 is dangerous and governments around the world are correct to insist on the accuracy of any widely distributed diagnostics tool.
In this situation, data is critical. Thankfully time and considerable levels of government backing is giving scientists and clinicians an opportunity to build their understanding and begin work on the optimisation and evaluation of antibody tests. This process does not happen overnight, but recent weeks have seen progress. Early results are beginning to emerge, with one example being the BioMedomics test I briefly highlighted in my previous blog. In the past two weeks the company has partnered with the global medical technology giant Beckon Dickinson & Co2 to further the manufacturing and distribution of the test and Harvard Medical School clinicians have since indicated positive preliminary clinical findings on the test, with full publication to follow soon.
Biomedomics is also participating in the large test evaluation scheme being coordinated by the Foundation for Innovative New Diagnostics (FIND), a healthcare non-profit based in Geneva, in collaboration with the World Health Organisation and Geneva’s world-renowned University Hospital. Since my last blog this has progressed swiftly (for an excellent introductory article on this important programme of work, see “Testing the Tests: Which COVID-19 Tests Are Most Accurate?”), with the organisation prioritising the evaluation of rapid tests. 27 antibody tests and 5 antigen tests (which, unlike antibody tests, are designed to identify the direct presence of the virus in the sample) are currently under evaluation,3 and the results will undoubtedly contribute to the body of data on COVID-19 diagnosis and help to prioritise optimisation of the most promising tests; I will share the initial data in a future blog.
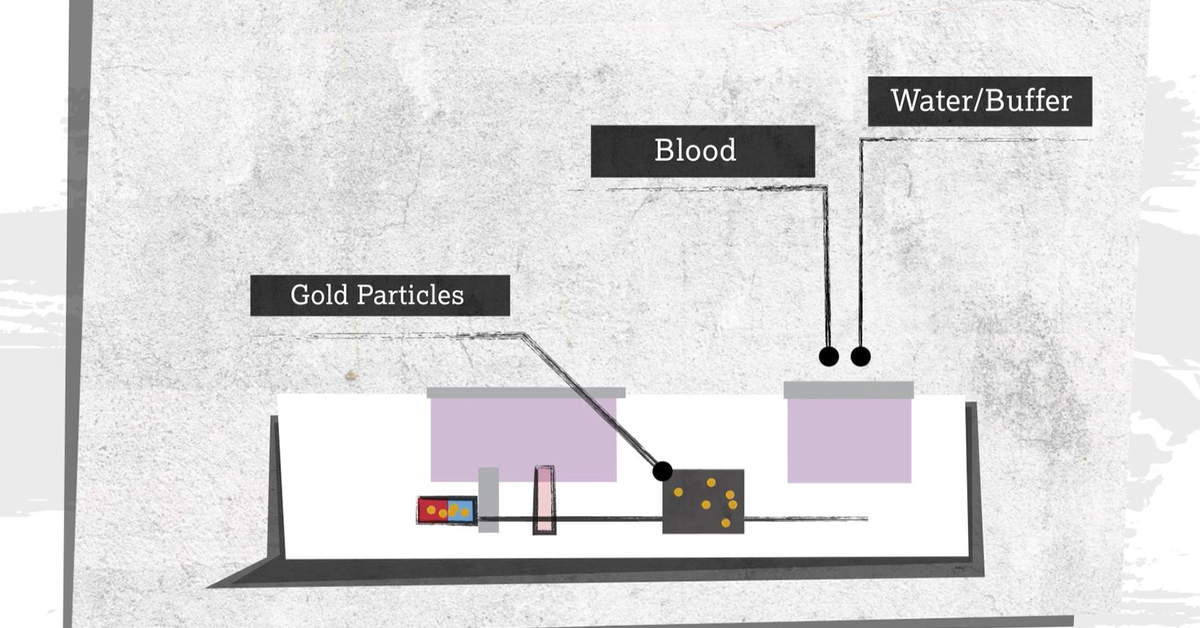
The vast majority of these 32 tests contain gold; the metal is critical to rapid antibody and antigen tests alike. If you are interested in how these tests work check out the World Gold Council’s recently published animation below.